© 2001 Subandiyono Posted 18
May 2001 [rudyct]
Science
Philosophy Student Paper (PPs 702)
Graduate School, Institut
Pertanian Bogor
Indonesia.
Instructors:
Prof Ir Rudy
C Tarumingkeng, MF, PhD (Principal)
Prof Ir Zahrial
Coto, MSc, PhD
BIOLOGY
OF RABBITFISH IN RELATION TO
MARICULTURE
PROSPECTS IN INDONESIA
BY:
SUBANDIYONO
{NRP.: P.19600002.AIR}
mailto:subandiyono@hotmail.com
PREFACE
The paper entitled BIOLOGY OF RABBITFISH IN RELATION TO MARICULTURE PROSPECTS IN INDONESIA is one of the assignments in Philosophy of Science (PPS 702) and was made to accomplish of the subject.
The paper reviewed and discussed the
biologically potential aspects of rabbitfish, one of the economically important
marine fish, to mariculture, including the advantage and disadvantage
characteristics of the fish. Most of the
information was based on the references; however, recent experiences obtained
by the author were included. Classification,
description and distribution {ONTOLOGY}, the reasons why the fish was becoming
more important economically and biologically to mariculture in Indonesia
{EPISTEMOLOGY}, the expectations on the mariculture development of the fish in
the future {TELEOLOGY}, and the contradictive situations so that mariculture
activities of the fish were not well developed in Indonesia {CAUSALITY} were
described. The purpose of these
maricultural perspectives urgently to be developed {AXIOLOGY} was also
implied.
I am grateful to Dr. Kok Leong
Wee for his critical review of the paper and to Professors Rudy C. Tarumingkeng, PhD. and Zahrial Coto,
PhD. for their supervisions during the class sessions.
Last but not
least, any critiques to improve the paper are welcome.
The author
INTRODUCTION
Indonesia is an
archipelago in which the seawater area is much bigger than the freshwater area,
i.e. about 70%:30%, respectively.
However, in terms of commercial fish farming, mariculture is not
developed yet. One of the marine fish
species that has great commercial potential for intensive culture is rabbitfish
or siganids (locally named beronang or samadar). Even though this fish has been popular as
excellent seafood with a good price, there is little mass production. This is due to difficulties in larval rearing
and the high larval mortalities that occurred during the first week from
hatching (Ayson and Lam, 1993; Duray et al., 1994; Subandiyono et al.,
1998; 1999; 2000).
Rabbitfish are
widely distributed in the Indo-Pacific region, from the east coast of Africa to
Polynesia, and from southern Japan to northern Australia. They can also be found in the eastern
Mediterranean (Duray, 1990). They are, therefore, a fairly cosmopolitan
group of fishes (Lam, 1974).
There are 26 known
species of rabbitfish. This paper will
only focus on those species that are important for the future of mariculture in
Indonesia.
BIOLOGICAL ASPECTS
A. CLASSIFICATION
The biological
classification of the rabbitfish described by Duray (1990) is as follows:
Phylum: Chordata
Subphylum: Vertebrata
Grade:
Pisces
Class:
Osteichthyes
Subclass:
Acteropterigii
Infraclass:
Neopterigii
Division:
Halecostomi
Subdivision:
Teleostei
Superorder:
Acanthopterigii
Order:
Perciformis
Family:
Siganidae
Genus:
Siganus (Teuthis)
Includes to this species are: S. argenteus (Quoy
& Gaimard), S. canaliculatus (Park), S. corallinus, S.
fuscescens (Houttunyn), S. guttatus (Bloch), S. luridis (Ruppell),
S. spinus, (Linnaeus), S. vermiculatus (Cuvier &
Valenciennes).
B. DESCRIPTION
In general,
rabbitfish can be described morphologically as follows: 1) the body shape is
compressed; 2) the body is protected by smooth and small cycloid scales; 3) the
snout resembles that of a rabbit; 4) the terminal-small mouth possesses small
teethes; 5) the linea lateralis is simple; and 6) the number of spines
at the dorsal, anal region and ventral is 13, 7 and 2, respectively (Fig.
1). Species are identified based on
their color and behavior.

Figure 1. A 32-cm body length-broodstock of S.
guttatus
C. GONADAL DEVELOPMENT
The time required
for one cycle gonadal development varies between species (e.g. S.
canaliculatus requires about 4.5 months).
Also, this is affected by several environmental conditions such as: 1)
photoperiod (e.g. 18 h light : 6 h dark retards gonadal maturation of S.
canaliculatus) (Lam and Soh, 1975); 2) quantity and quality of diet (e.g.
females of S. guttatus fed with commercial diet containing 43% protein
spawned monthly for 11 months; when lecithin, cod liver oil, or both were added
to the diet, spawned occurred for at least 4 consecutive months) (Hara et al.,
1986a); 3) temperature (e.g. rapid gonadal development usually occurs when the
water temperature range from 25-30 ΊC); and 4) lunar cycle (e.g. gonads of S.
canaliculatus mature during new moon).
The development of
gonads can be identified at the following stages, each characterized by the
size of the ovary (Table 1).
Table 1. Development
of gonads in relation to size of ovary of the rabbitfish (Alcala and Alcazar,
1979 in Duray, 1990)
|
STAGE |
SIZE OF OVARY (mM) |
STAGE OF OOCYTE DEVELOPMENT |
|
I |
14 70 |
Chromatin nucleolus and early perinucleolar oocytes |
|
II |
14 238 |
Late perinucleolar and yolk vesicle oocytes |
|
III |
56 350 |
Yolk vesicle and primary yolk oocytes |
|
IV |
210 364 |
Secondary and tertiary yolk oocytes |
|
V |
266 406 |
Mature oocytes |
|
VI |
336 420 |
Eggs |
|
VII |
14 70 |
Resting or desorbing oocytes |
D. FECUNDITY AND EGG PROPERTIES
In general,
absolute fecundity is higher in larger fish.
Female S. canaliculatus of 11.1 11.5 cm body length produces 166,000
650,000 eggs (Lam, 1974; Woodland, 1979; Tseng and Chan, 1982), while at 21.6
27.3 cm body length (or about 166 346 g body weight) may produce 348,000
1,339,000 eggs (Basyari et al., 1988).
A 400-g S. guttatus produces 0.8 million eggs, while a 520-g fish
produces 1.2 million. Newly caught S.
vermiculatus with 25 30 cm TL (total body length) produce 200,000
fertilized eggs (Popper and Gundermann, 1976).
When ripe, the egg
is: small, spherical, demersal and strongly adhesive (Subandiyono et al.,
2000; Fig. 2), except in the case of S. argenteus that are free-floating
and non-adhesive (Lam, 1974). This
sticky layer enables the eggs to attach to any type of substrate, whether
floating or static, as spawning sites.
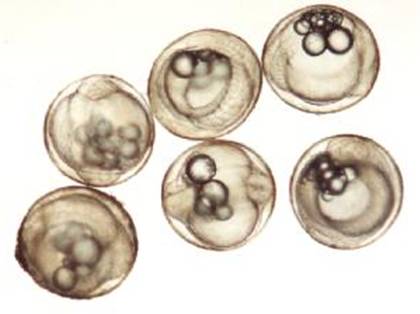
Figure 2. Newly fertilized
eggs (±24-h embryos) of S. guttatus
The time required
for egg incubation is slightly different depending on the species, water
temperature, and egg diameter (Table 2).
In general, bigger eggs and lower water temperature result in a
relatively longer period of incubation.
For example, fertilized eggs with diameter 0.42 0.70 mm require 18
35 h at 22 30ΊC, except for S. canaliculatus that needs 62 h (Table
2).
Table 2. The relationship between egg size of rabbitfish, incubation period, water temperature and salinity
Species |
Egg Diameter (mm) |
Incubation Period (h) |
Water Temp. (ΊC) |
Water Salinity (ppt) |
Type of Incubator Used |
Refs. |
|
S. argenteus |
0.62 0.68 |
- |
24 25 |
40 |
a) |
1) |
|
S. canaliculatus |
0.51 |
30 |
27 29 |
20.9 32 |
60-l tanks |
2) |
|
S. guttatus |
0.55 0.55 0.54 0.59 0.55 0.57 56 - 58 |
20 26 20 18 20 18 25 24 |
26 30 26 29 26 28 27 30 27 28 |
Ambient 32 33 31 34 33 32 |
Tanks 500-l tanks Tanks b) 1-l beakers |
3) 4) 5) 6) 7) |
|
S. luridus |
0.50 0.63 |
- |
24 25 |
40 |
a) |
1) |
|
S. rivulatus |
- |
29 30 |
25.5 27 |
- |
2-l beakers |
8) |
|
S. vermiculatus |
0.56 |
24 |
30 |
- |
c) |
9) |
|
|
|
|
|
|
|
|
a): 200-l cylindrical tanks with conical bottom and 1000-l cylindrical flat bottom tanks; b): 5-t rectangular concrete tank; c): 100-l plastic tanks and nylon mesh hatching baskets.
1) Popper et al., 1979; 2) Westernhagen and Rosenthal, 1976; 3) Juario et al., 1985; 4) Bagarinao, 1986; 5) Hara et al., 1986a; 6) Hara et al., 1986b; 7) Ayson and Lam, 1993; 8) Popper et al., 1973; 9) Popper et al., 1976
FOOD AND FEEDING HABITS
A. FIRST-FEEDING LARVAE
Rabbitfish larvae hatch
with non-functional eyes and mouth; and the onset of feeding will commence
after these organs become functional.
Meanwhile, the larvae continue to consume the endogenous nutrient supply
for their growth (Table 3).
Table 3. The transition from endogenous to exogenous
feeding of rabbitfish larvae (after Kohno et al., 1988)
|
Phase |
Period |
Remarks |
|
I |
Hatching 15 h TAH |
Rapid growth due to rapid yolk resorption |
|
II |
15 50 h TAH |
Slow growth and organogenesis based mainly on yolk energy |
|
III |
50 70 h TAH |
Slow growth based on energy of yolk, oil globule and
exogenous food |
|
IV |
70 90 h TAH |
Slow growth based on energy from oil globule and exogenous
food |
|
V |
90 120
h TAH |
Slow growth based on energy from oil globule and certain
amount of feeding |
|
VI |
120
150 h TAH |
Accelerated growth and effective swimming and feeding
based only on exogenous food |
|
VII |
Beyond
150 h TAH |
Same mode as in the preceding but with accelerated
increase in food consumption |
Bagarinao (1986)
and Subandiyono et al. (1999; 2000) found that the eyes of larvae S.
guttatus become fully pigmented and the mouth opens at 36 h TAH (time after
hatching), while complete yolk resorption occurs at 72 h TAH. Thus, as the time required from the initial
feeding to the oil globule exhaustion is relatively short (i.e. about 36 h),
the provision of suitable food during this critical period is crucial for the
larvae to survive further. A delay in
the initial feeding beyond 24 h of eye pigmentation and mouth opening (i.e.
after 60 h TAH) may be fatal for the larvae (i.e. causes 50% mortality), while
starved larvae will die after 88 h TAH (Bagarinao, 1986).
Chlorella,
rotifers, Brachionus sp. (in which the size less than 90 μm) can be
used to improve the survival rate of the first feeding larvae (Hara et al.,
1986a). Copepod nauplii may be more
suitable as its size is smaller than Brachionus. However, Chlorella, Tetraselmis
and Isochrysis as the sole food for the larvae will not support life
beyond 4 days TAH (Duray, 1990; Subandiyono et al. 1998; 1999;
2000).
Besides phytoplankton and zooplankton, an artificial diet
can be added (e.g. after 20 days TAH) when the larvae grow bigger (Bryan and
Madraisau, 1977; Juario et al., 1985; Hara et al., 1986a). The use of enriched live food to feed
rabbitfish larvae may improve the larval performance. Sorgeloos et al. (1988) reported that
HUFA-fortified Artemia increased the growth of S. guttatus
larvae.
The feeding behavior of rabbitfish larvae changes distinctly
during metamorphosis (Bryan and Madraisau, 1977). At the first stage (i.e. pre-metamorphosis)
they are carnivorous fish. They become
omnivorous, then herbivorous at the last stage (post- metamorphosis). Furthermore, larvae of S. guttatus
exhibit diurnal feeding pattern. It has
been shown that the percentage of larvae with food in the gut decreases in the
evening and reaches zero at 22:00 hrs.
The time of active feeding (i.e. 50% of larvae with food in the gut)
shifts earlier in the day with larval age.
B. JUVENILES
Whereas rabbitfish
larvae are zooplankton feeders, the juveniles are primary herbivorous. Thus, the juveniles have a thick stomach wall
and long intestine with a large surface area (Basyari et al.,
1988). As with the larvae, the juveniles
feed actively during the day to the evening and become inactive at night
(Duray, 1990; Popper and Gundermann, 1975).
Light may be used to attract the juveniles when collecting them from the
wild. Usually, filamentous green algae
are required as a lure (Ben-Tuvia et al., 1973; Bwathondi, 1982), and
then a net was used to scoop the fish.
In captivity,
rabbitfish required high dietary protein and energy for better growth. Parazo (1990) did an experimental feeding
trial on juveniles cultured in 250-l tanks for 8 weeks. Six semi-purified diets comprising 3 levels
of protein (i.e. 25, 35, and 45% of dry matter) each at 2 levels of estimated
energy (i.e. 3161 and 3832 kcal/kg diet) were fed to juveniles at a stocking
density of 80 fish/tank. It was
concluded that the growth increased with increasing dietary protein and energy. Also, there is a positive correlation between
the body weight gain and dietary protein-to-energy ratio (P/E). For diets with the same energy content but
different dietary protein levels, higher P/E rations yielded better growth
rate. Conversely, lower P/E rations also
resulted in better growth rate for the diets containing the same protein levels
but with higher energy content. Parazo
(1990) suggested that the fish fed low-energy diets had insufficient total
energy intake to satisfy their requirement as compared to those fed high-energy
diets. However, Parazo (1990)
recommended that a diet with high energy content (i.e. 3832 kcal/kg diet) and
medium protein content (i.e. 35%) to be the most economical diet for juveniles S.
guttatus.
Lichatowich et
al. (1984) used a moistened mixture of soy meal (53%), fish meal (14%),
maize (15%), flour 15%) and vitamin-mineral premix (3%) to feed 3 g-juvenile
rabbitfish reared in 10-m3 cages for about 5 months. This investigations resulted in the
non-significant growth rate for the juveniles stocked in different density,
either monoculture or polyculture system with other fish (e.g. sea bream, Crenibus
crenibus).
C. ADULT
In the wild, adult
rabbitfish consume seagrass (e.g. Enhalus sp., Padina sp., Gelidium
and Sargassum halophyla) or filamentous algae (e.g. Chaetomorpha
sp., Enteromorpha sp. and Cladophoropsis sp.) (Basyari et al.,
1988), whereas in captivity they take any types of food including the seagrass,
filamentous algae, fish meals, shrimp meals, cassava flour or pelleted diet
(Subandiyono, 1998; 1999; Subandiyono et al., 1996; 1997). However, adequate protein content in the diet
or mixed-diet is needed as the fish (e.g. S. canaliculatus) gives poor
growth rate when fed low protein diets or just seaweeds (Bwathondi, 1982).
Feeding habits of
adult fish may be influenced by the food available in the area where they live,
as they are opportunistic feeders. The
analysis of gut content showed that the algae preferred by captive fish were
not always those found in greatest quantity in the gut of wild fish
(Westernhagen, 1973; 1974). Juveniles
and adult S. spinus, juveniles S. argenteus, S. guttatus, S.
virgatus, and S. canaliculatus prefer Enteromorpha sp. in the
laboratory but take this only in small amounts in nature (Tsuda and Bryan,
1973; Westernhagen, 1973; 1974). However,
Enteromorpha sp. is important in the diet of S. rivulatus and S.
argenteus lived in the Elat Gulf, Middle East (Lichatowich et al.,
1984). This phenomenon indicates that
the differences of food preference may be related to the algal availability and
other factors in the area.
D. BROODSTOCKS
The quality of
diet for the broodstocks is an important factor for the survival performance of
the larvae (Duray et al., 1994; Subandiyono, 1999; Subandiyono et al.,
1998; 1999; 2000). Also, the age of spawner
cause the fertilization and hatching rates and larval quality to decline. Juario et al. (1985) found that the
percentage of larval survival rate at the first experiment varies between 6.3
37.4%. By using the same broodstocks,
this value decreased to 0.9 9.0% for the next year and 0.7 2.0% for the
next 2 years. Hormonal treatment for
broodstocks prior to spawning has been investigated to improve larval
performance. An experiment done by
Ayson and Lam (1993) showed that larvae from females treated with 10 and 100
μg T4-thyroxine/g fish tended to be longer and somewhat better
survivors.
MARICULTURAL ASPECTS IN INDONESIA
Recently,
rabbitfish are becoming more important as a mariculture product. In terms of maricultural purposes, rabbitfish
possess both desirable and undesirable characteristics.
A. DESIRABLE
CHARACTERISTICS:
1.
The fish are an
excellent food with high market value (Duray, 1990). In Indonesia they cost about twice of
milkfish, a staple fish for meals. As a
traditional dish during Chinese New Year, their price can be even more
expensive at such time (i.e. may be twice as much as normal price).
2.
Due to the beauty of
their color, some of them (e.g. S. magnificus and S. vulpinus)
are also sold live as an aquaria fish to America and Europe. They fetch more than US$ 100/pair (Woodland,
1979).
3.
The marketable size is
relatively small (i.e. varies about 100 300 g, depending on species). Therefore, a fast turnover for farmers, as
they can grow fish to this size quickly.
4.
Rabbitfish are able to
take an artificial diet (Bwathondi, 1982; Juario et al., 1985; Hara et
al., 1986a; Subandiyono, 1999). This
encourages mass production using intensive culture system.
5.
The fish can be
cultured in monoculture or polyculture system with milkfish (Chanos chanos),
mullets (Mugil and Liza spp.) or seabass (Lates calcarifer)
(Lichatowich and Popper, 1975; Bagarinao, 1986), without affecting growth.
6.
The fish spawn easily,
whether naturally or by using hormonal treatment (Ayson and Lam, 1993;
Subandiyono et al., 1999; 2000).
7.
The fecundity is
relatively high, approximately 0.8 million eggs for 400 g fish and 1.2 million
for 520 g fish (depending on size and species) (Popper and Gundermann, 1976).
8.
Even though the larvae
are very fragile, they can be transported for 2 days using simple equipment
(Basyari et al., 1988).
9.
Large numbers of
rabbitfish juveniles can be collected from coastal waters during certain
seasons (Lam, 1974), for instance in northern coast of Java in the beginning of
rainy seasons.
10.
The juveniles and
adults most species occupy shallow water (Lam, 1974; Popper et al.,
1979). Therefore, in terms of commercial
farming, they dont require a deep cage.
11.
They inhabit different
types of habitat (e.g. coral reef, sandy and rocky bottom with or without
vegetation, lagoons, river estuaries, and mangrove swamps) (Lam, 1974; Popper
and Gundermann, 1975; Woodland and Randall, 1979).
12.
Rabbitfish are able to
tolerate a wide range of salinity and temperature (5 50 ppt and 23 32 ΊC)
with a preference range of about 10 35 ppt and 26 30ΊC.
13.
Rabbitfish can be used
to control the growth of filamentous algae if they are stocked in shrimp ponds
and tropical oyster or clam culture (Chen, 1990).
14.
They can be used as a
bait to catch tuna (Duray, 1990).
B. UNDESIRABLE
CHARACTERISTICS:
1.
In general, they grow
slowly but mature early. For example,
sexual maturity for male S. guttatus is about 10 months with the size
about 19 cm, while the female maturity is reached after 12 months with the size
about 21 cm or about 200 g. The growth
rate decreases after attaining sexual maturity.
2.
Even though natural or
induced spawning is not a problem, especially in S. guttatus (Hara et
al., 1986a; Duray et al., 1994; Subandiyono et al., 2000),
mass juvenile production is still limited.
3.
The time required from
the initial feeding to the exhaustion of endogenous nutrient supply is
relatively short, i.e. about 36 h TAH (Duray and Kohno, 1988).
4.
The larvae have a
relatively small mouth gape at the first opening, i.e. about 125 μm (Duray
and Kohno, 1988). Therefore, they need
small size feeds.
5.
The fish are difficult
to handle due to the poisonous spines that may induce severe headaches
(Herzberg, 1973).
6.
The sex is difficult to
distinguish except during the breeding season (Duray, 1990).
7.
It is difficult to
differentiate between the species due to only a few morphological
differences. Therefore, the
identification relies only on the coloration of live fish, habitat, and behavioral
characteristics (Woodland and Randall, 1979).
CONCLUSIONS
As the
biologically aspects and feeding habits of rabbitfish vary between species,
special attention to one or few species that have commercial potential for
mariculture is needed. Considerations
include the ease of spawning, with or without hormonal treatment. The chosen species should be fecund, fast
growing, hardy and suited to intensive culture.
The important species for mariculture in Indonesia are S.
vermiculatus, S. guttatus, S. canaliculatus and S. javus.
These fish have
potential prospects to be cultured commercially in Indonesia as they are suited
to the local growing conditions and are a recognized delicacy with a high
market value. Other factors which
encourage the farming of rabbitfish in Indonesia are: 1) the fish can be grown
using a simple floating cage (e.g. made of bamboo which is abundant in
Indonesia; 2) sites suited to this type of fish farm are easy to find; 3) many
types of commercial diet for fish have been produced in a large quantity; and
4) labor cost is relatively cheaper.
Therefore, rabbitfish farming has tremendous potential in Indonesia.
REFERENCES
Ayson, F.G. and Lam, T.J., 1993. Thyroxine injection of female rabbitfish (Siganus guttatus) broodstock: changes in
thyroid hormone levels in plasma, eggs, and yolk-sac larvae, and its effect on
larval growth and survival. Aquaculture,
109: 83-93.
Bagarinao, T., 1986.
Yolk resorption, onset of feeding and survival potential of larvae of
three tropical marine fish species reared in the hatchery. Mar. Biol., 91: 449-459.
Basyari, A., Danakusumah, E., Philip, T.I., Pramu, S.,
Musthahal dan Isra, M., 1988. Budidaya
ikan beronang (Siganus sp.). INFIS, No. 60, Direktorat Jenderal Perikanan,
31 hal.
Ben-Tuvia, A., Kissil, G.W. and Popper, D., 1973. Experiments in rearing rabbitfish (Siganus
rivulatus) in seawater. Aquaculture,
1: 359-364.
Bryan, P.G. and Madraisau, B.B., 1977. Larval rearing and development of Siganus lineatus (Pisces, Siganidae) from hatching through
metamorphosis. Aquaculture, 10(3):
243-252.
Bwathondi, P.O.J., 1982.
Preliminary investigations on rabbitfish, Siganus canaliculatus, cultivations in Tanzania. Aquaculture, 27(2): 205 - 210.
Chen, L.C., 1990. Aquaculture
in Taiwan. Fishing News Books, Oxford,
USA, 273 p.
Duray, M.N., 1990. Biology and culture of siganids. Aquaculture Department, SEAFDEC, Philippines, 47 p.
Duray, M. and Kohno, H., 1988. Effects of continuous lighting on growth and
survival of first-feeding larval rabbitfish, Siganus guttatus. Aquaculture, 72: 73-79.
Duray, M., Kohno, H. and Pascual, F., 1994. The effect of lipid-enriched broodstock diets
on spawning and on egg and larval quality of hatchery-bred rabbitfish (Siganus guttatus). The Philippine Scientist, 31: 42-57.
Hara, S., Duray, M., Parazo, M. and Taki, Y., 1986a. Year-round spawning and seed production of
the rabbitfish, Siganus guttatus. Aquaculture, 59: 259-272.
Hara, S., Kohno, H. and Taki, Y., 1986b. Spawning behavior and early life history of Siganus
guttatus in the laboratory.
Aquaculture, 59: 273-285.
Herzberg, A., 1973.
Toxicity of Siganus luridus (Ruppell) on the Mediterranean coast
of Israel. Aquaculture, 47: 53-59.
Juario, J.V., Duray, M.N., Duray, V.M., Nacario, J.F. and
Almendras. J.M.E., 1985. Breeding and
larval rearing of the rabbitfish Siganus
guttatus (Bloch). Aquaculture, 44:
91-101.
Kohno, H., Hara, S., Duray, M. and Gallego, A., 1988. Transition from endogenous to exogenous
nutririon sources in larval rabbitfish Siganus guttatus. Nippon Suisan Gakkaishi, 54(7):
1083-1091.
Lam, T.J., 1974.
Siganids: their biology and mariculture potential. Aquaculture, 3: 325-354.
Lam, T.J. and Soh, C.L., 1975. Effect of photoperiod on gonadal maturation
in the rabbitfish Siganus canaliculatus
(Park,1797). Aquaculture, 5: 407-410.
Lichatowich, T., Al-Thobaity, S., Arada, M. and Bukhari, F.,
1984. Growth of Siganus rivulatus reared in sea cages in the Red Sea. Aquaculture, 40: 273-275.
Lichatowich, T. and Popper, D., 1975. Report on the growth of rabbitfish in fish
ponds in Fiji. Aquaculture, 5: 211-212.
Parazo, M.M., 1990.
Effect of dietary protein and energy level on growth, protein utilization
and carcass composition of rabbitfish, Siganus
guttatus. Aquaculture, 86: 41-49.
Popper, D., May, R.C. and Lichatowich, T., 1976. An experiment in rearing larval Siganus vermiculatus (Valenciennes) and some observations on its
spawning cycle. Aquaculture, 7:
281-290.
Popper, D. and Gundermann, N., 1975. Some ecological and behavioral aspects of
siganid populations in the Red Sea and Mediterranean coasts of Israel in
relation to their suitability for aquaculture.
Aquaculture, 6: 127-141.
Popper, D. and Gundermann, N., 1976. Asuccessful spawning and hatching of Siganus
vermiculatus under field conditions.
Aquaculture, 7(3): 291-292.
Sorgeloos, P., Leger, P. and Lavens, P., 1988. Improved larval rearing of European and Asian
seabass, seabream, mahi-mahi, siganid and milkfish using enrichment diets for Brachionus and Artemia. World Aquacult.,
19(4): 78-79.
Subandiyono, 1998.
Peranan rumput laut, pakan buatan, dan campurannya pada pertumbuhan dan
pematangan gonad ikan beronang (Siganus
sp.). Majalah Ilmu Kelautan, 10:
82-86.
Subandiyono, 1999.
Growth of rabbitfish, Siganus
sp., in a captivity fed by diets containing different level of
soy-lecithin. Journal of Coastal
Development, 2(3): 419-425.
Subandiyono, 1999.
Kebutuhan asam lemak-W3 dan W6 dalam pakan induk ikan beronang (Siganus sp.). Majalah Penelitian, 41: 91-100.
Subandiyono, Hermawan, I. dan Widianingsih, 1996. Peranan penggantian rumput laut dengan pakan
buatan terhadap bioenergetika ikan beronang (Siganus sp.). Lemlit-Universitas
Diponegoro, 50 hal.
Subandiyono, Hermawan, I. dan Widianingsih, 1997. Aplikasi bioteknologi untuk ikan beronang (Siganus sp.) dalam kaitannya dengan
prospek budidaya laut di Indonesia.
Tahap Akhir: Pemanfaatan berbagai sumber bahan pakan lokal pada
pengadaan induk menggunakan bak semi-terkontrol (Tahun I). Lemlit-Universitas Diponegoro, 48 hal.
Subandiyono, Kokarkin, C. dan Hastuti, S., 1998. Paket teknologi formulasi pakan induk ikan
beronang (Siganus sp.) guna
meningkatkan kualitas telur (Tahun I).
Abstrak-Hasil Hasil Penelitian Tahun 1997/1998, Lemlit, UNDIP, pp.:
98-99.
Subandiyono, Kokarkin, C. dan Hastuti, S., 1999. Paket teknologi formulasi pakan induk ikan
beronang (Siganus sp.) guna
meningkatkan kualitas telur. Tahun II. Lemlit-Universitas Diponegoro, 81 hal.
Subandiyono, Kokarkin, C. dan Hastuti, S., 2000. Paket teknologi formulasi pakan induk ikan
beronang (Siganus sp.) guna
meningkatkan kualitas telur. Tahun III. Lemlit-Universitas Diponegoro, 102 hal.
Tseng, W.Y. and Chan, K.L., 1982. The reproductive biology of the rabbitfish in
Hong Kong. J. World Maricult. Soc., 13:
313-321.
Tsuda, R.T. and Bryan, P.G., 1973. Food preference of juvenile Siganus rostratus (S. argenteus) and S. spinus in Guam. Copeia, 3: 604-606.
Westernhagen, H.M., 1973.
The natural food of the rabbitfish Siganus
oramin and S. striolata. Mar. Biol., 22: 367-370.
Westernhagen, H.M., 1974.
Food preferences in cultured rabbitfishes (Siganidae). Aquaculture, 3: 109-117.
Westernhagen, H.M. and Rosenthal, H., 1976. Induced multiple spawning of reared Siganus oramin (Schneider) (Siganus canaliculatus Park). Aquaculture, 7: 193-196.
Woodland, D.J., 1979. Rabbitfishes neglected in Australia are important food fish in tropical countries. Aust. Fish., 38(6): 21-23.
Woodland, D.J. and Randall, J.E., 1979. Siganus
puelloides, a new species of rabbitfish from the Indian Ocean. Copeia, 3: 390-393.